
Voluntary Recall: HeartSine® samaritan® PAD SAM 350P/360P/450P
This device recall notification is being issued to alert customers with HeartSine samaritan PAD SAM 350P, 360P, and 450P devices of a potential device malfunction issue. Out of an abundance of caution, Stryker is completing a voluntary recall of these devices.
Is Your HeartSine AED Under Voluntary Recall?
Check Multiple Serial Numbers
NOTE: If you have serial numbers affected by the voluntary recall, you MUST submit the form below or you will receive additional follow-up from our team.
Please complete the form below and we’ll help you take the next step:
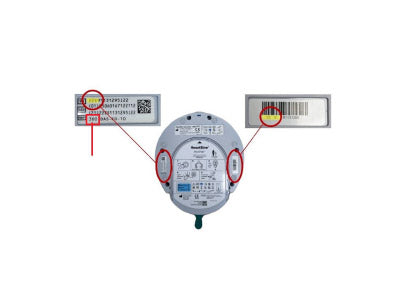
Locating the Serial Number
- Look for a barcode label or a sticker with numbers — the serial number contains a combination of numbers and letters (e.g., 24G1234567B12).
- It’s located near the center edges the back panel, or next to the pad cartridge compartment.
- The serial number is also listed on the original packaging and the product documentation if you still have it.
How May Your Device Be Affected
-
Product Issue
It was determined during extensive quality testing that a manufacturing process issue related to a circuit board component may impair the device’s ability to function or cause failure. This failure could occur at any point when the device is holding a charge in preparation to deliver therapy, while delivering a shock, or after shock delivery. The device becomes inoperable after the failure occurs.
-
Potential Risks
If this issue occurs, the device may fail to deliver the intended therapy during use, potentially leading to a delay in treatment or no treatment being delivered during use. The issue was observed during quality testing, not during patient use. There have been no adverse events reported related to this product issue.
HeartSine Recall FAQs
What is the issue?
A manufacturing process change that was implemented to achieve compliance with European environmental regulatory requirements has impacted the quality of a component of the device’s electrical circuit, causing devices to experience failure during extensive quality testing.
We are initiating a voluntary field action.
What is the likelihood of this issue occurring?
The likelihood of this issue occurring is low. This issue was identified through quality testing and not through patient use. There have been no adverse events reported related to this product issue.
I have received notifications on this device previously. Is this the same, or different?
This is the first notification issued by Stryker regarding this component issue. This issue was identified due to a change in a manufacturing process change that was implemented to achieve compliance with European environmental regulatory requirements.
What am I supposed to do with my device?
We recommend that customers keep their devices in service until replacement devices are available.
When will replacement devices be available?
Alternate devices are not being offered at this time as the affected devices can be kept in use until a HeartSine replacement is available. The benefits of attempting to use an AED during sudden cardiac arrest are greater than the risk of not having one to use or not attempting to use one in an emergency.
Can I have a different AED while I wait for my replacement?
Stryker is working on implementing updates for the HeartSine devices and currently anticipates returning to production in Q4 2025. There are regional regulatory requirements that may add additional time to availability. Once shipping of products resumes, we anticipate it could take several months to complete all the impacted product replacements in your region.
What if I don’t feel comfortable continuing to use my device?
You may use an alternate AED if available; however, the likelihood of this issue occurring is low. This issue was identified through quality testing and not through patient use. There have been no adverse events reported related to this product issue. If this issue occurs during patient use, please continue CPR and seek an alternative device.We recommend that customers keep their devices in service until replacement devices are available.
I don’t want to wait for a replacement device; I want a refund.
The probability of the issue occurring is low. Based on the testing data analysis, completed in March 2025, there is a 99.98% likelihood of a successful delivery of at least one shock. At this time, Stryker will be providing replacement devices when they become available. Please continue to keep the device in service in the meantime. Stryker does not intend to provide a refund at this time since we will be providing replacement devices as soon as they are available.
Stryker is working on implementing updates for the HeartSine devices and currently anticipates returning to production in Q4 2025. There are regional regulatory requirements that may add additional time to availability.
Once shipping of products resumes, we anticipate it could take several months to complete all the impacted product replacements in your region.
We thank you sincerely for your help and support in submitting your response by August 31, 2025. We regret any inconvenience that may be caused and would like to reassure you that we are committed to meeting our high internal quality standards and your expectations.
The U.S. Food and Drug Administration has been notified of this action. Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
